LISBON, Portugal — A new nasal spray treatment is showing promise for patients with hard-to-treat cases of depression. The neurological disorder is a global mental health issue, but for some, it’s an especially persistent problem. This stubborn form of the condition, known as treatment-resistant depression (TRD), continues to affect patients in spite of taking medications that typically work for others.
“TRD is defined as the persistence of depressive symptoms despite adequate courses of at least two different antidepressant medications,” explains Albino Oliveira-Maia, head of the Champalimaud Foundation’s Neuropsychiatry Unit and the study’s national coordinator in Portugal, in a media release.
Tackling TRD has been a significant hurdle. Data from the National Institute of Mental Health (NIMH) indicates that while one-third of those with depression find relief from their initial treatment, only 10 to 15 percent find relief after three rounds of treatment. Given this challenge, there’s a push for more effective treatment options.
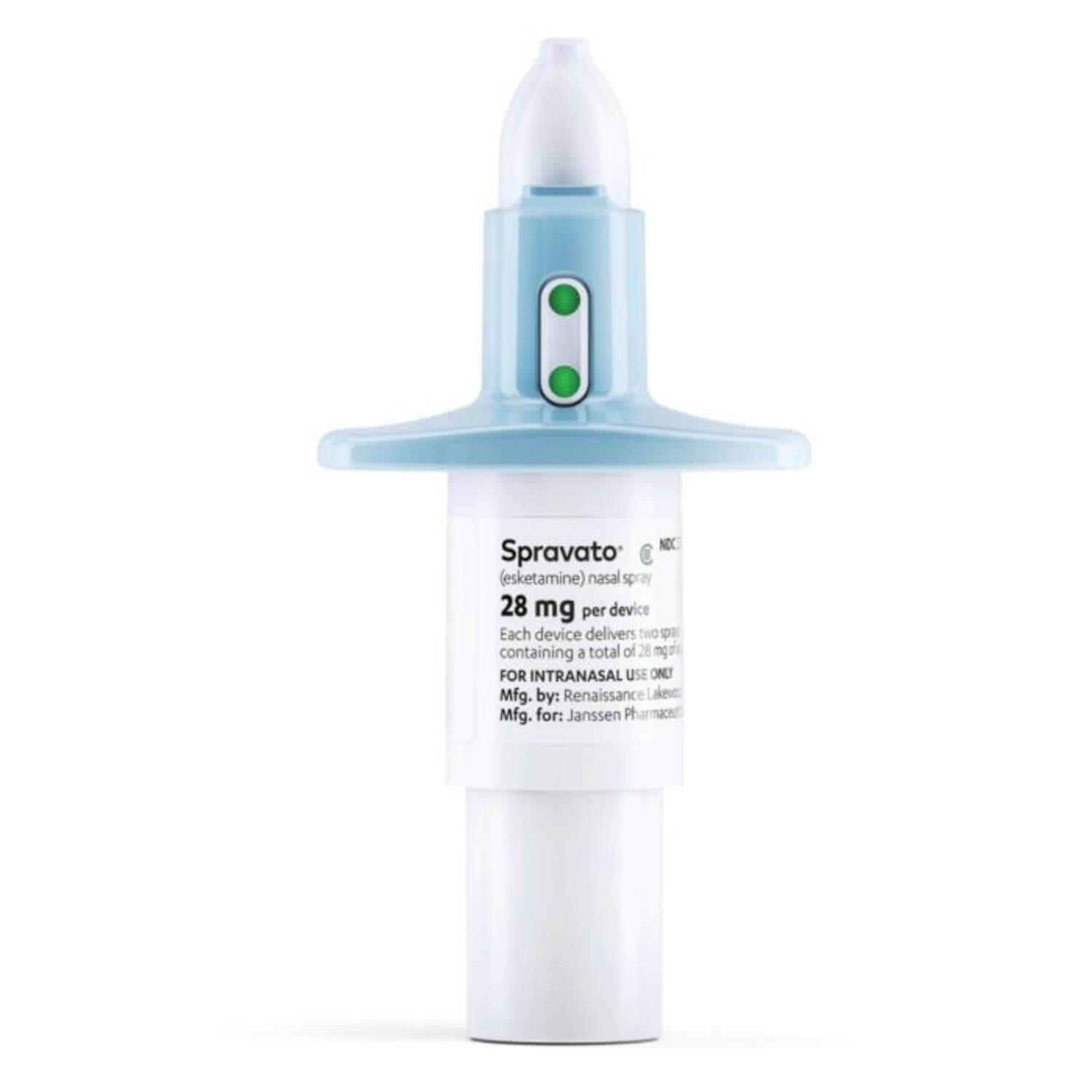
Recently, the medical community has been buzzing about esketamine nasal spray, developed by Janssen. It has outperformed placebo (a substance with no therapeutic effect) in numerous clinical trials and has received approval from both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Why the excitement over esketamine?
“While there are many treatments available for depression, there is a paucity of drug options tailored for TRD,” says Oliveira-Maia. “Moreover, in order to guide clinicians and patients in their decision-making, and to be adopted by health insurance companies and governments, drug manufacturers need to demonstrate a distinct advantage over existing treatment modalities, underscoring the rationale for this study.”
The study pitched esketamine against quetiapine XR, a drug initially meant for conditions like schizophrenia. This drug has recently been green-lit for use in hard-to-treat depressive episodes.
“Quetiapine is currently one of the few alternative medications approved as an add-on for patients with a major depressive episode and inadequate response to ongoing antidepressant treatment,” notes Oliveira-Maia.
Over 800 patients were considered for the study, with 600 fitting the strict TRD criteria. These participants were divided into two groups. One group took quetiapine XR at home, and the other was administered esketamine in a hospital. Both groups also continued taking a standard antidepressant.
“The study spanned 32 weeks, which is longer than typical trials,” says Oliveira-Maia. “This allowed us to gauge both short-term and long-term treatment outcomes. Throughout this time, we closely monitored participants’ responses, side effects, and the medications’ overall efficacy.”

(© RawPixel.com
– stock.adobe.com)
What were the results?
At the eight-week mark, the study found that 27.1 percent of the esketamine group achieved remission (or, essentially, a symptom-free state), compared to 17.6 percent in the quetiapine XR group. At the end of the study (32 weeks), of those who achieved remission by week 8, 21.7 percent from the esketamine group maintained it, compared to 14.1 percent in the quetiapine group.
Both treatments had low instances of severe side-effects. Yet, more patients on esketamine reported minor side-effects.
“This was anticipated, given esketamine’s dissociative properties,” says Oliveira-Maia. “Interestingly, the rate of patients discontinuing treatment due to side effects was actually lower for esketamine NS than for quetiapine XR, which suggests that while esketamine NS may have more side effects on paper, those caused by quetiapine were less tolerable.”
While the findings are a beacon of hope for those with TRD, Oliveira-Maia urges caution.
“The real challenge is shifting from research to policy,” says Oliveira-Maia. “The impact of esketamine NS can only be realized if patients can readily access it.”
In places like Portugal, access to proven TRD treatments is limited.
“Continued research and strong advocacy are needed to make sure treatments reach the patients who need them,” says Oliveira-Maia.
Looking to the future, Oliveira-Maia remains hopeful, aiming to delve deeper into improving remission rates and exploring other treatments like transcranial magnetic stimulation (TMS).
“Our future research endeavors aim to pinpoint predictive markers for treatment responsiveness,” adds Oliveira-Maia. “Additionally, we want to investigate ways to improve and sustain remission rates, including the potential role of psychotherapy. TMS is also high on our list for future exploration. However, scientific progress must be matched with proactive policy measures and concrete governmental action. Ultimately, our goal is to construct a healthcare landscape where patients are not relegated to subpar, non-evidence-based treatments due to lack of access to more effective options.”
The study is published in the New England Journal of Medicine.
You might also be interested in:
- Depression and anxiety may be the first symptoms of multiple sclerosis
- Ordinary nasal spray shows promise of treating sudden rapid heartbeat emergencies at home
- These 7 healthy habits help prevent depression — and scientists now know why